What Is A Hypothesis On Natural Selection Affects Skin Color
- Review
- Open Access
- Published:
Adaptation of human skin color in various populations
Hereditas book 155, Article number:1 (2018) Cite this commodity
Abstract
Background
Skin color is a well-recognized adaptive trait and has been studied extensively in humans. Understanding the genetic basis of adaptation of skin color in diverse populations has many implications in human evolution and medicine.
Word
Impressive progress has been made recently to identify genes associated with pare color variation in a wide range of geographical and temporal populations. In this review, we hash out what is currently known about the genetics of skin color variation. We enumerated several cases of skin color adaptation in global modernistic humans and archaic hominins, and illustrated why, when, and how skin color adaptation occurred in different populations. Finally, we provided a summary of the candidate loci associated with pigmentation, which could be a valuable reference for further evolutionary and medical studies.
Decision
Previous studies by and large indicated a complex genetic mechanism underlying the skin color variation, expanding our understanding of the role of population demographic history and natural selection in shaping genetic and phenotypic diversity in humans. Future work is needed to dissect the genetic architecture of pare color adaptation in numerous ethnic minority groups around the world, which remains relatively obscure compared with that of major continental groups, and to unravel the exact genetic basis of peel color adaptation.
Background
Since modernistic humans ventured out of Africa ~100,000 years ago, they spread across continents into a variety of habitats, from tropical zones to the arctic, and from lowlands to highlands. During migration, selective pressures in local environments (due east.g., the cold climate, hypoxia, and endemic pathogens), together with random drift, have resulted in population-specific genetic variants, which further influenced variable phenotypes, such as lactose tolerance, height, immune system, and metabolic efficiency.
Pare color variation is i of the most striking examples of human phenotypic multifariousness. It is dominated by melanin, a pigmentation located in the base of operations of the epidermis and produced by melanocytes. Melanin has two forms, pheomelanin (yellowish-reddish) and eumelanin (blackness-brownish). The sometime is mainly accumulated in the calorie-free-complexioned people, while the latter is generally produced in the dark-complexioned people [1,2,3,4,5]. In add-on, the number and size of melanin particles differ among individuals, and is even more than important than the proportions of the two forms of melanin in the conclusion of human being skin color [5]. Other skin-related factors, e.1000., keratin, also contribute to skin colour variation [6, 7].
In global populations, skin color is highly correlated with latitude, and fundamentally, the distribution of ultraviolet (UV) radiation (Fig. 1). Populations closer to the equator tend to have dark pare for protection against UV, since overexposure to UV may decrease folic acid levels [8, 9] and cause skin cancer [ten,xi,12,xiii]. The lighter skin in populations at higher latitudes is underlying pick to maintain vitamin D photosynthesis, which is a UV-dependent procedure [14, 15].
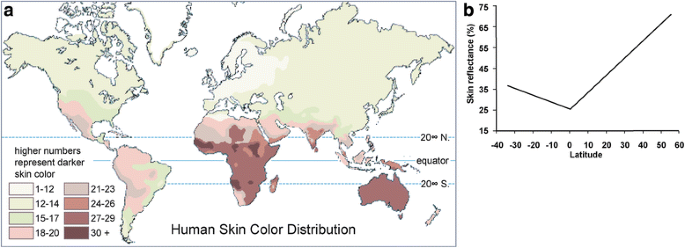
Correlation betwixt skin color and latitude (from Barsh (2003) [5]). (a) A map of human peel color distribution. (b) A plot of skin reflectance confronting latitude
Although UV has been assumed to be a driving force for the evolution of human skin colors, agreement the exact genetic mechanism of selection would be crucial to reconstruct human being evolutionary history and elucidate the microevolution of adaptive traits. Describing a full picture of regional skin color adaptation in humans would be challenging because it includes non simply the genes identified to be under option, simply also the extent to which these genes could explain phenotypic variation, the interactions and joint furnishings of genes, and the style they react to the external environments. In this article, we reviewed several cases of skin color adaptation in various populations of modern humans and archaic hominins. These cases show the similarities and differences of mechanisms of pare colour adaptation across populations, and provide some insights into man evolutionary history.
Peel color adaptation in modernistic Eurasians
In Europeans, SLC24A5 and SLC45A2 [sixteen,17,18,19] are ii golden genes related to the evolution of the light skin colour. SLC24A5 encodes the NCKX5 protein, which is a member of the transmembrane protein family and regulates the calcium concentration in the melanosome [xvi]. This gene has been confirmed to affect pigmentation in zebrafish and mice [16, 20]. Especially, the derived allele of rs1426654 in SLC24A5 was institute to be about fixed in Europeans, but almost missing in populations without any European beginnings (Fig. ii) [21]. A 78-kb haplotype around SLC24A5, which is in linkage disequilibrium with rs1426654, was also identified to accrue in Europeans [22]. A similar pattern can be observed at rs16891982 in SLC45A2 [23], which has been reported to be associated with pigmentation in several species, e.g., mice, fish, birds, and horses [24,25,26]. Other variants in this gene, including rs26722, rs2287949, and rs40132, were too shown to exist coloration-associated in Europeans [23, 27, 28]. Another of import pigmentation-related cistron identified in European is MC1R [29,30,31]. This gene is expressed in melanocytes and plays a key role in controlling the switch from pheomelanin to eumelanin [31]. The pigmentary phenotypes associated with MC1R has been studied in a wide range of animals [32,33,34]. Many variants have been identified in MC1R, such as rs1805007, rs1805008, and rs3212357 [35, 36], despite its modest size (951 bp). Other of import European-specific loci include rs1393350 in TYR, rs2733831 in TYRP1, and rs1900758 in OCA2 [17, 28, 37,38,39]. The derived allele frequencies at these loci are high in Europeans just depression in Africans and East Asians, which could be a clear signal of positive selection in Europeans, as indicated by statistical analysis [40].
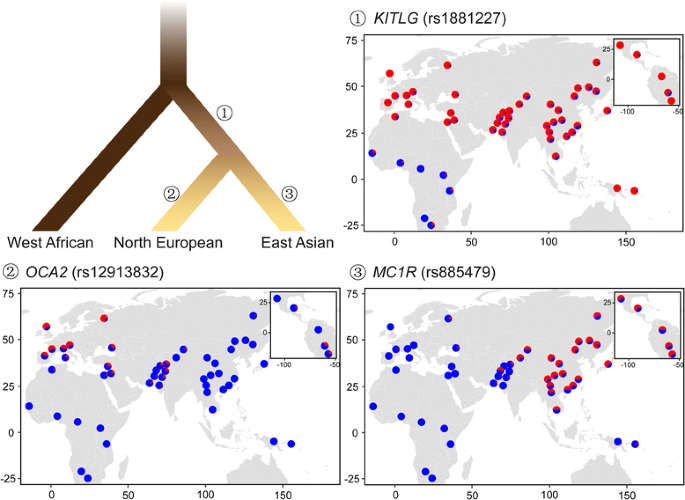
Evolutionary model of human pigmentation in three continental populations. The rooted tree shows the genetic phylogeny of human being populations from Africa, North Europe and East Asia, with the colors of the branches roughly indicating the generalized peel pigmetation level of these populations (adapted from McEvoy et al. (2006) [39]). Genetic loci reported to be under positive selection in the common ancestor of modern Eurasians are represented by rs1881227 in KITLG, and those independently evolved in Europeans and Due east Asians, indicating possible convergent evolution, are represented by rs12913832 in OCA2 and rs885479 in MC1R, respectively. The maps of allele frequency were fatigued using R (version iii.2.1, https://www.r-projection.org), based on these loci in 53 global populations provided by the Homo Genome Diversity Panel CEPH (HGDP, http://www.hagsc.org/hgdp/index.html). Blue and red colors denote the ancestral and derived alleles, respectively
Genes involved in the peel color accommodation in East Asians are not that well studied compared to the long list of adaptive genes identified in Europeans. Notable examples include OCA2 and MC1R. Each harbors several non-synonymous mutations, eastward.chiliad. rs1800414 and rs74653330 in OCA2, and rs885479 in MC1R [40,41,42,43], which exhibit loftier derived allele frequencies in East Asians, but depression derived allele frequencies in Europeans and Africans (Fig. two). The OCA2 protein is thought to be a mature melanosomal membrane poly peptide [44], with a potential role in protein transportation into melanosomes [45]. The East Asian-specific variant of rs1800414 was first reported in an exome sequencing study aiming to figure out albinism-related variants [46]. The derived allele at rs1800414 was thought to contribute to the pare lightening in an association study of Han Chinese, which measured the skin colour of individuals using the melanin index [47]. Some other non-synonymous variant in OCA2, rs74653330, has also been confirmed to be pigmentation-related in an association written report of Japanese [48]. Additional examples of E Asian-specific pigmentation-associated alleles include rs10809814 in TYRP1 and rs1407995 in DCT [40, 49], both of which show differentiation between Asians and non-Asians [47], and strong signals of positive selections in Asians [43, 49].
Despite distinct genes and variants under respective local adaptations in Europeans and Due east Asians, some genes have derived alleles reaching high frequencies in both continental groups. For instance, KITLG exhibits a selective sweep in not-Africans [50,51,52]. This gene is widely expressed in multiple tissues, including the peel, and functions in organ morphogenesis and jail cell proliferation. The Kit-ligand encoded by KITLG is known as the steel factor and plays a crucial office in the normal development and maintenance of the melanocyte lineage in adult skin [53]; this has been proved in human being, fish, and mice [54,55,56]. The furnishings of this gene on pigmentation have also been confirmed in a series of association studies [57,58,59,sixty]. 1 of the primal variants is rs642742, which is located at 326 kb upstream to the transcription start site of KITLG. At this variant, the ancestral allele frequency is over 90% in Africans, comparable to the derived allele frequency in Europeans and Due east Asians (Fig. 2). Similar patterns were observed in other genes, e.g., ASIP and BNC2 [39].
Two models of the evolutionary architecture of homo pigmentation were proposed on the ground of the in a higher place results and other related studies (Fig. ii). 1 is a convergent evolution model [17, 40, 43, 49], suggesting that depigmentation has, to some degree, evolved independently in Europeans and East Asians, equally different genes and variants have been suggested to explicate the lite skin and positive selection in these two continental groups. A contempo study estimated the time of selective sweeps for the European-specific pigmentation variants to be around 11,000–nineteen,000 years ago, later on the divergence of Europeans and Asians [61]. An alternative model fits for the shared selective sweeps of Europeans and East Asians, which could mayhap occur in proto-Eurasians. The onset of the sweep was estimated to exist approximately 30,000 years ago, right after the "Out-of-Africa" migration, but earlier than the European-specific evolution on pigmentation [61]. The coexistence of these two models suggests a complex evolutionary history of pare colour in modern humans.
Another inkling of the complex genetic basis of peel colour evolution is the allelic heterogeneity observed in a single factor, like OCA2 and MC1R. In each of these genes, some alleles are specific to Europeans, whereas others are specific to Asians, although they all have been proved to be depigmentation-related. In add-on, OCA2 provides evidence of independent sweeps too as convergent evolution in Europeans and Asians. Since results were obtained from studies using different samples, data, and methods, there could be some confounding factors leading to these different observations. Yet, more importantly, peel colour is a circuitous trait that could not exist simply explained past a unmarried factor or variant; rather, information technology is likely to involve a huge network of genes and phenotypes. For example, ASIP, an adaptive pigmentation gene in populations with European ancestry [62, 63], encodes the agouti signaling poly peptide, which blocks MC1R in the eumelanin synthesis in response to the UV-induced Deoxyribonucleic acid damage [40]. In the melanin production, TYR acts as the catalyzer of the key initial step, and its stability is maintained by TYRP1 and DCT.
In addition, scans for selection on peel pigmentation indicate two dissimilar selection behaviors interim on de novo mutations and standing variations, respectively. Some variants, represented by rs1805007 and rs1805008 in MC1R (in Europeans) and rs1800414 in OCA2 (in Asians), but show derived alleles in populations under positive selection at these loci, from which nosotros could conjecture that they are new mutations that appeared later on modern humans settled in Europe or Asia. In dissimilarity, some variants, such as rs3212357 in MC1R (under positive choice in Europeans), present depression frequencies in Africans. Regardless of possible mutation events and genetic migrate in African populations, it is more likely that the derived allele at this locus has presented for some time before they became favored. Like cases take been found in the loftier-distance accommodation of Tibetans and the immunity adaptation in some modern homo populations, and even in the development of pigmentation phenotypes in not-human species [56, 64].
Skin color accommodation in the admixed populations
Admixed populations, the hybrid offspring of two previously isolated populations, may provide important insights in understanding the genetics of geographical variation for ii reasons. First, the loci underlying phenotypic differences in ancestral populations are also overlapping the highly informative markers of ancestry, which makes the admixed populations particularly useful for tracing population history. 2nd, the admixed populations usually take a broad range of variations regarding some specific phenotypes, which may increase the power of locating genes associated with complex traits/diseases after controlling potential population stratification.
Despite these advantages, admixed populations have rarely been considered in studies of homo pigmentation variation. Electric current studies investigating pigmentation genes in admixed populations mainly involved those with African and European ancestry, such as African Americans, European Africans, and Latin Americans, since their ancestral populations are substantially differentiated in pare color. The ancestral genetic makeups differ among these iii populations. African-Americans obtained the largest genetic contribution (~80%) from the African ancestry [65], Latin American mestizos have the least proportion of African beginnings (~10%) [66, 67], while in European Africans, the genetic components inherited from Europeans (~42%) and Africans (~58%) are comparable [68]. Uniquely in the Latin Americans, a considerable proportion of Native American ancestry (~45%) exists [66, 67]. Moreover, on the individual level, the proportion of each ancestry exhibits a large variance in each admixed population. For instance, the fraction of European ancestry varies from 2% to 98% among African American individuals [65]. The big variance of peel color in admixed individuals could result from their highly diverse genetic makeup, as a substantial correlation has been observed between ancestry proportion and skin color [68,69,70].
Multiple well-known candidate genes for pigmentation in Europeans have also been identified by admixture mapping (Fig. 3) or association studies in admixed populations. For instance, TYR, conveying a non-synonymous exchange rs1042602 (S192Y), was identified in African Americans [69] and European Africans from Republic of cape verde [68]. Variants in ASIP, such as rs6058017, which has been found to occur at different frequencies in global populations [63], were likewise reported to be associated with nighttime hair and brown optics in European Americans [71], African Americans [62] and Brazilians [72]. Furthermore, KITLG showed stiff signals of selective sweep in African Americans [51], with a significant preference to homozygotes of the African-specific allele (bequeathed allele) at rs642742 in individuals with high melanin index (dark pare) [69]. Similar cases include rs1426654 in SLC24A5 in European Africans [68] and Latin Americans [72], and rs35395 in SLC45A2 in European Africans [68].
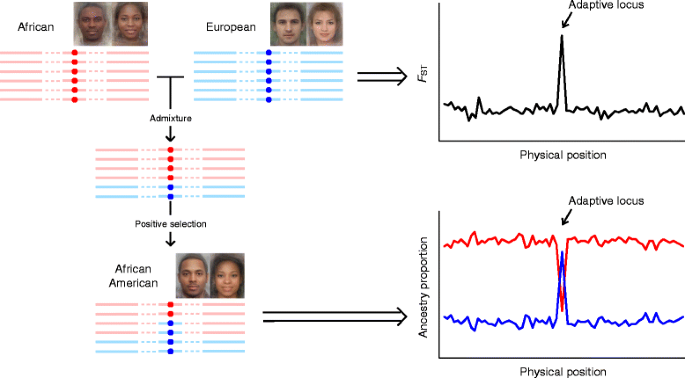
A framework of admixture mapping to detect positive selection. The boilerplate faces of African, European, and African America were downloaded from http://www.mediadump.com/hosted-id167-average-faces-from-around-the-world.html#.WLkMU-kfU1A
Still, some studies reported discrepant results. The correlation between Native American ancestry and skin pigmentation reported in a Hispanic population [73] was not observed in a group of Puerto Rican women [70]. 1 of the key unmarried nucleotide polymorphism loci (SNPs) in OCA2, rs1800404, showed a pregnant effect on skin pigmentation when analyzing African Americans and a combined population of African American and African-Caribbean, but was absent-minded in an independent analysis of the African-Caribbean area samples [69]. It is possible that unlike genetic mechanisms of pare colour variation exist in various populations, simply cautions should exist taken regarding detailed information in the data, such every bit sample size and the ancestral populations selected for analyzing the admixed populations, which could lead to biased results [67, 69].
The identification of genetic determinants of natural variation of pare pigmentation was besides conducted in other admixed populations. One successful case is a genome-wide association study of a population of South Asian descent [74], in which polymorphisms in SLC24A5, TYR and SLC45A2 showed significant associations with the melanin content in skin. The light peel alleles in South Asian could peradventure be inherited from their European ancestors [75], who initially arrived at this region around 3500–4000 years ago along with Indo-European language expansion [76], followed past recent colonization in the last few centuries. In add-on, Central Asia and Southeast Asia are home to various admixed populations, which are likewise of great potential in the study of pare color adaptation. Admixed population analyses may greatly enrich our understanding of skin color variation in modernistic human populations.
Skin colour accommodation in the ancient populations
The ancient populations in different areas around the world have many implications for human being evolutionary history. They have been regarded as the early settlers in respective areas. Despite having been alloyed past their surrounding agriculturalists to some extent, some aboriginal people have preserved their traditional livelihoods as hunter-gatherers, equally well as their original physical traits – dark pare, brusque stature, and curly hair.
The hunter-gatherer populations with dark skin, short stature and curly pilus have attracted much attending (Fig. 4a). The genetic mechanism underlying the shared phenotypes amongst these geographically afar populations (collectively chosen Negritos or Pygmies), from Central Africa, the Andaman Islands, Southeast Asia and Oceania, are still controversial; for example, whether they were the common descent from a pre-Neolithic substrate of humanity or a issue of convergent evolution [77, 78]. To date, near genetic studies on this issue have focused on height [78,79,lxxx,81]. I study provided clues for convergent evolution from the view of peel pigmentation adaptation by analyzing MC1R multifariousness in the Melanesians [82]. This study showed that the ancestral haplotypes of MC1R are not highly conserved betwixt Northern Island Melanesians and Africans, although both populations live in the high UV region, which is in dissimilarity to previous findings based on very limited samples [30, 83]. Besides, a non-synonymous polymorphism, rs2228479, shows enriched derived alleles specifically in East Asians, simply is not significantly associated with peel or pilus pigmentation in Melanesians. Actually, the Melanesian population exhibits hit skin pigmentation variation [84], and consistently, some variants have been identified to be region-specific, which could partly explain this phenotypic variation. A notable example is a non-synonymous variant, rs387907171, in TYRP1 [85]. It is restricted to the Solomons and parts of the Bismarck Archipelago, and might contribute to the 'blond hair' in this region [85, 86]. These results emphasize the complex genetic architecture of pigmentation phenotypes, and also highlight the role that population history (eastward.m., the complex population history of the Southwest Pacific [87,88,89]) can play a role in influencing phenotypic diversity. Pare pigmentation studies on other modernistic ancient populations (besides Melanesians) are scarce, except for one investigating the Senoi population (an indigenous population) from the Malay Peninsula, which is an admixture of the Negrito (night-skinned) and the southern Mongoloid from Indo-China (yellow-brown-skinned), and has a wide peel colour spectrum [90]. The authors of this study found that despite the low derived allele frequency, the A111T mutation (rs1426654) in SLC24A5 is significantly associated with the light skin in Senoi, which was suspected to result from the admixture of the Mongoloid and S Asians.

Skin color of aboriginal people in the Equatorial zone and the Arctic. (a) Skin colour comparison between Bateq (a subgroup of Negrito) and Malay from Peninsular Malaysia. (b) Skin color comparison between Inuit and Swedish from similar latitudes. Portraits of Malay and Swedish individuals are provided by the Joshua Project (http://joshuaproject.internet), the Bateq portrait is from http://world wide web.businessinsider.my/, and the Inuit portrait is from http://world wide web.arcticphoto.co.uk/
Another interesting result concerning human skin color adaptation comes from the arctic people. The Inuit people, in far Due north Eastern asia and the American Subarctic, have yellowish-brown skin despite the far northern latitude at which they live, different other populations living at the same latitude, such as the Swedes and Finnish (Fig. 4b). This makes the Inuit population an exception of the latitude-correlated distribution of peel color. One possible reason is that the dark pare could protect the Inuits from the severe UV exposure considering of the long daylight hours in wintertime and high levels of UV reflection from the snow. While the nighttime skin is a disadvantage for vitamin D production, enough of vitamins including vitamin D could be compensated from their diets [91, 92]. Another cause could be the founder effect of the aboriginal Eastward Asian ancestry of the Inuits, who have inhabited the chill region since nearly 5000 years ago, and had college melanin production than the European ancestry. Notwithstanding, very few genetic studies have been conducted to determine the genetic basis of dark skin in arctic populations.
Pare colour adaptation in the ancient hominins
The dark skin in modern humans was established effectually 1.2 meg years ago, driven by the loss of body hair after divergence from apes, presumably to protect confronting UV-induced damages [13, 93,94,95,96]. Then, when did mod Eurasians first to depigment? The studies on skin colour adaptation summarized above are based on modern population genetic data, which may suffer from limited temporal resolution caused past the population demographic history, and insensitivity to selection interim on standing variations [97]. The advent of aboriginal DNA analyses makes it possible to directly discover the development processes, and thus would facilitate our understanding of this key question.
A study on the genomes of Anatolian Neolithic farmers in West Eurasia (6500–300 BC), who are probably the source population of the offset European farmers, suggests that the light skin color has been evolved since at least 6500–4000 years ago [98]. Several popular genes identified in mod Eurasians, e.g., SLC45A2, GRM5 and HERC2/OCA2 showed potent signal of selection in these ancient samples. This conclusion is supported by another study based on the Eneolithic (6500–5000 BP) and Bronze Age (5000–4000 BP) samples, representing the early European farmers or late hunter-gatherers in central Europe [99]. One possible motivation of the skin depigmentation in prehistoric Eurasia is agriculturalization, which led to a switch from vitamin D-rich hunter-gatherer diet to a vitamin D-poor agriculturalist diet, together with the increased danger of folic acid deficiency at higher latitudes [14, 100]. Moreover, the selective pressures accept kept operating for a long time after they initiated the accommodation of pare colour, as some ancestral pigmentations alleles were identified in a Mesolithic European (7000 BP), and some adaptive alleles under selection in the aboriginal Eurasians are all the same evolving in modernistic humans [98, 99, 101].
Recent studies on archaic hominins (east.g., Neanderthals, an extinct hominid grouping living in Eurasia ~400,000–28,000 years agone [102]) farther improved our understanding of skin color development in modern humans. Neanderthals met modern humans in the Heart East ~60,000–50,000 years ago, and contributed to about one–4% of mod homo genomes [103,104,105]. Some pigmentation-associated genes are identified in the introgressed haplotypes from Neanderthals in modern Eurasians, such as POU2F3, BNC2 and MC1R [106, 107]. Specifically, the introgressive alleles were reported to result in light skin color, suggesting an 'adaptive introgression' strategy of homo peel color accommodation. Other introgressive genes related to pare phenotypes include HYAL genes, which are associated with cellular responses to UV and are nether strong positive selection in East Asians [108], and those involved in keratin filaments formation [109]. Although these genes are not straight determinants of skin pigmentation, they, like those pigmentation-related genes, possibly helped modern humans adapt to non-African environments.
When drawing conclusions of adaptive introgression, we are actually claiming that Neanderthals could be light-complexioned. This inference is but based on some pigmentation-associated genes or alleles identified in existing modern human populations, since visible phenotypes of Neanderthals and other extinct species are not available. Yet, when using another priory genes as potential clues, different results tin can be obtained. For instance, the derived state of MC1R, which is responsible for pale pare, presents in Neanderthal individuals from Italy and Spain only is missing in Croation Neanderthals and Denisova [110], suggesting skin colour variation in the archaic hominins. In addition, the light skin in Neanderthals and modern Eurasians could too outcome from convergent evolution, rather than adaptive introgression [111].
The hypothesis of adaptive introgression seems to predate when mod human became pale – long before the late Mesolithic age, as Neanderthals went extinct around 28,000 years agone. Nevertheless, we should reconsider whether the genes affecting skin color in primitive hominins indeed determined skin color in mod humans. Even if this is the case, information technology is also possible that mod human being retained these introgressive variants until they showed some phenotypic effects nether some specific strong selective pressures. Thus, more information resources and analyses are necessary to address this upshot in the future.
Selection coefficient and upshot size
As one of the most obvious changes in the environment after modern human migrated out of Africa to higher latitudes, UV has exerted considerable selective pressures on human peel pigmentation, which tin be reflected past selection coefficients of the pigmentation-related genes. The estimation of selection coefficients largely depends on the genes considered and the methodologies. Beleza et al. estimated the coefficient of selection at several loci representing SLC24A5, SLC45A2, TYRP1, and KITLG [61]. For example, the estimates are 0.05/0.04 for SLC45A2 and 0.16/0.08 for SLC24A5 under a ascendant/an condiment model of inheritance in Europeans. Meanwhile, López et al. reported the selection coefficient of a variant in SLC45A2 to exist 0.01–0.02 in a S European populations [112]. These estimations are comparable to the selection coefficients inferred directly from serially sampled data at HERC2, SLC45A2, and TYR, ranging from 0.02–0.1 [99]. The selection coefficients estimated for pigmentation genes are best understood in the context of estimates for other recently selected loci. The selection advantages are inferred to exist 0.01–0.08 for LCT, a cistron strongly associated with lactase persistence in populations with European ancestry [113, 114], 0.019–0.048 for G6PD, a gene conferring malaria resistance in African populations [115], 0.03–0.19 for EDAR associated with the increased scalp pilus thickness and inverse tooth morphology in the Han Chinese [116], and 0.0004–0.0023 for EGLN1 and EPAS1 gene regions contributing to the high-altitude adaptation in Tibetans [117]. The option coefficients for pigmentation genes are among the most strongly selected genes in the human genome, indicating a astringent selective pressure caused by UV or some other environmental changes in non-African regions.
Although a large number of genes accept been identified to contribute to skin colour variation, how much could they explicate the skin color variation in modern humans? Is there a gene or variant that has a ascendant effect on the skin colour? Some genes could possibly play a major role in determining skin color in specific populations. For instance, the lite pare variant at rs1426654 in SLC24A5 could explain 22–32% of the variance of the melanin alphabetize in Southward Asian [75] and 25–38% in African-American and African-Caribbean populations [118]. Additionally, the derived allele at rs642742 in KITLG may account for lightening of a person'due south skin by 6 to 7 melanin units, nearly 1/5 of the overall pare reflectance divergence betwixt Due west Africans and Europeans (xxx melanin units) [56]. Withal, there are relatively more genes and variants with smaller effects. I of the key variants in OCA2, rs1800414, could explain around 4% of the pigmentation variation in East Asian populations [47]. In Southward Asians, rs16891982 in SLC45A2 and rs1042602 in TYR account for iii.vi% and 2.five% skin color variation, respectively, much less than the effect size of rs1426654 in SLC24A5 [74]. The inheritance style of peel pigmentation follows an additive model, or at least an incomplete additive model [16, 17, 47, 56, 75].
Conclusions
Overall, human being peel color is a highly variable and complex trait as a consequence of strong selection pressure and is controlled past multiple genetic loci (summarized in Table 1). Skin color adaptation is a complex process because dissimilar populations have shared and independent genetic mechanisms involving a large number of genes with dissimilar result advantages on the phenotype. Skin colour adaptation is also a long evolutionary procedure influenced by various historical, even pre-historical, population genetic events. Electric current studies provide comprehensive insights into the natural selection procedure and mechanisms of man skin color variation. A richer resource of high-coverage whole-genome sequences and phenotype information may provide opportunities to further speculate an accurate model of genetic compages and gene-environs effects, and accelerate our agreement of skin pigmentation in certain small-scale ethnic groups, such every bit hunter-gatherers and highlanders. We believe that these studies may greatly enrich our noesis of human evolution history and elucidate the genetic basis of complex traits in humans.
References
-
Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Paint Prison cell Res. 2003;16:523–31.
-
Hennessy A, Oh C, Diffey B, Wakamatsu Chiliad, Ito S, Rees J. Eumelanin and pheomelanin concentrations in human being epidermis before and after UVB irradiation. Pigment Cell Res. 2005;18:220–3.
-
Thody AJ, Higgins EM, Wakamatsu K, Ito Southward, Burchill SA, Marks JM. Pheomelanin equally well every bit eumelanin is present in human epidermis. J Invest Dermatol. 1991;97:340–iv.
-
Riley PA. Melanin. Int J Biochem Cell Biol. 1997;29:1235–9.
-
Barsh GS. What controls variation in man pare color? PLoS Biol. 2003;1:e27.
-
Tennessen JA, Akey JM. Parallel adaptive divergence among geographically diverse homo populations. PLoS Genet. 2011;seven:e1002127.
-
Gautam P, Chaurasia A, Bhattacharya A, Grover R. Indian genome variation consortium, Mukerji M, et al. population diversity and adaptive evolution in keratinization genes: impact of environment in shaping skin phenotypes. Mol. Biol. Evolution. 2015;32:555–73.
-
He YY, Wang XC, Jin PK, Zhao B, Fan X. Complexation of anthracene with folic acid studied by FTIR and UV spectroscopies. Spectrochim Acta A Mol Biomol Spectrosc. 2009;72:876–9.
-
Juzeniene A, Stokke KT, Thune P, Moan J. Pilot study of folate status in healthy volunteers and in patients with psoriasis before and afterwards UV exposure. J Photochem Photobiol B Biol. 2010;101:111–six.
-
Armstrong BK, Kricker A. The epidemiology of UV induced peel cancer. J Photochem Photobiol B Biol. 2001;63:8–18.
-
De Gruijl FR, Van Kranen HJ, Mullenders LHF. UV-induced DNA harm, repair, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol B Biol. 2001;63:nineteen–27.
-
Brenner K, Hearing VJ. The protective role of melanin confronting UV impairment in human skin. Photochem Photobiol. 2008;84:539–49.
-
Greaves M. Was skin cancer a selective force for blackness pigmentation in early on hominin evolution? Proc R Soc B. 2014;281:20132955.
-
Jablonski NG, Chaplin G. Homo peel pigmentation equally an adaptation to UV radiation. Proc Natl Acad Sci U South A. 2010;107(Suppl 2):8962–8.
-
Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiations: more than merely the effects of vitamin D? Nat Rev Immunol. 2011;11:584–896.
-
Lamason RL, Mohideen MA, Mest JR, Wong Air-conditioning, Norton HL, Aros MC, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Scientific discipline. 2005;310:1782–36.
-
Norton HL, Kittles RA, Parra Eastward, McKeigue P, Mao Ten, Cheng K, et al. Genetic evidence for the convergent evolution of lite skin in Europeans and East Asians. Mol Biol Evol. 2007;24:710–22.
-
Soejima Grand, Tachida H, Ishida T, Sano A, Koda Y. Bear witness for recent positive pick at the human AIM1 locus in a European population. Mol Biol Evol. 2006;23:179–88.
-
Ginger RS, Askew SE, Ogborne RM, Wilson Due south, Ferdinando D, Dadd T, et al. SLC24A5 encodes a trans-Golgi network poly peptide with potassium-dependent sodium-calcium substitution activeness that regulates homo epidermal melanogenesis. J Biol Chem. 2008;283:5486–95.
-
Vogel P, Read RW, Vance RB, Platt KA, Troughton K, Rice DS. Ocular albinism and hypopigmentation defects in Slc24a5−/− mice. Vet Pathol. 2008;45:264–79.
-
Soejima Yard, Koda Y. Population differences of two coding SNPs in pigmentation-related genes SLC24A5 and SLC45A2. Int J Legal Med. 2007;121:36–9.
-
Canfield VA, Berg A, Peckins S, Wentzel SM, Ang KC, Oppenheimer S, et al. Molecular phylogeography of a human autosomal skin color locus under natural selection. G3. 2013;iii:2059–67.
-
Cook AL, Chen W, Thurber AE, Smit DJ, Smith AG, Bladen TG, et al. Assay of cultured human melanocytes based on polymorphisms inside the SLC45A2/MATP, SLC24A5/NCKX5, and OCA2/P loci. J Invest Dermatol. 2009;129:392–405.
-
Fukamachi S, Shimada A, Shima A. Mutations in the gene encoding B, a novel transporter poly peptide, reduce melanin content in medaka. Nat Genet. 2001;28:381–5.
-
Mariat D, Taourit S, Guérin Yard. A mutation in the MATP gene causes the cream coat colour in the equus caballus. Genet Sel Evol. 2003;35:119–33.
-
Gunnarsson U, Hellström AR, Tixier-Boichard Chiliad, Minvielle F, Bed'hom B, Ito S, et al. Mutations in SLC45A2 cause plume color variation in craven and Japanese quail. Genetics. 2007;175:867–77.
-
Nakayama Thousand, Fukamachi S, Kimura H, Koda Y, Soemantri A, Ishida T. Distinctive distribution of AIM1 polymorphism among major human populations with different pare color. J Hum Genet. 2002;47:92–4.
-
Valenzuela RK, Ito South, Wakamatsu K, Brilliant MH. Prediction model validation: normal homo pigmentation variation. J Forensic Res. 2011;2:1000139.
-
Martínez-Cadenas C, López S, Ribas Thou, Flores C, García O, Sevilla A, et al. Simultaneous purifying selection on the ancestral MC1R allele and positive option on the melanoma-risk allele V60L in s Europeans. Mol Biol Evol. 2013;30:2654–65.
-
Harding RM, Healy East, Ray AJ, Ellis NS, Flanagan N, Todd C, et al. Evidence for variable selective pressures at MC1R. Am J Hum Genet. 2000;66:1351–61.
-
Rees JL, Flanagan Due north. Pigmentation, melanocortins and reddish hair. Q J Med. 1999;92:125–31.
-
Klungland H, Vâge DI, Gomez-Raya 50, Adalsteinsson South, Lien S. The role of melanocyte-stimulating hormone (MSH) receptor in bovine coat color determination. Mamm Genome. 1995;6:636–9.
-
Marklund L, Moller MJ, Sandberg K, Andersson L. A missense mutation in the cistron for melanocyte-stimulating hormone receptor (MC1R) is associated with the anecdote glaze color in horses. Mamm Genome. 1996;vii:895–nine.
-
Takeuchi Due south, Suzuki H, Yabuuchi M, Takahashi Due south. Possible involvement of melanocortin 1-receptor in regulating feather colour pigmentation in the craven. Biochim Biophys Acta. 1996;1308:164–8.
-
Sturm RA. GSTP1 and MC1R in melanoma susceptibility. Br J Dermatol. 2012;166:1155–6.
-
Tiosano D, Audi Fifty, Climer S, Zhang Due west, Templeton AR, Fernández-Cancio M, et al. Latitudinal clines of the human vitamin D receptor and pare color genes. G3. 2016;6:1251–66.
-
Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes, pigmentary phenotypes, and chance of skin cancer in Caucasians. Int J Cancer. 2009;125:909–17.
-
Myles S, Somel Chiliad, Tang Thou, Kelso J, Stoneking M. Identifying genes underlying skin pigmentation differences among man populations. Hum Genet. 2007;120:613–21.
-
McEvoy B, Beleza S, Shriver MD. The genetic compages of normal variation in human pigmentation: an evolutionary perspective and model. Hum Mol Genet. 2006;15:176–81.
-
Sturm RA, Duffy DL. Human pigmentation genes under ecology selection. Genome Biol. 2012;13:248.
-
Donnelly MP, Paschou P, Grigorenko E, Gurwitz D, Barta C, Lu RB, et al. A global view of the OCA2-HERC2 region and pigmentation. Hum Genet. 2012;131:683–96.
-
Yuasa I, Umetsu K, Harihara S, Kido A, Miyoshi A, Saitou Due north, et al. Distribution of two Asian-related coding SNPs in the MC1R and OCA2 genes. Biochem Genet. 2007;45:535–42.
-
Hider JL, Gittelman RM, Shah T, Edwards M, Rosenbloom A, Akey JMJ, et al. Exploring signatures of positive selection in pigmentation candidate genes in populations of East Asian ancestry. BMC Evol Biol. 2013;thirteen:150.
-
Sitaram A, Piccirillo R, Palmisano I, Harper DC, Dell'Angelica EC, Schiaffino MV, et al. Localization to mature melanosomes past virtue of cytoplasmic dileucine motifs is required for human OCA2 role. Mol Biol Jail cell. 2009;20:1464–77.
-
Hoyle DJ, Rodriguez-Fernandez IA, Dell'Angelica EC. Functional interactions between OCA2 and the protein complexes BLOC-i, BLOC-ii and AP-3 inferred from epistatic analysies of mouse coat pigmentation. Pigment Cell Res. 2011;24:275–81.
-
Lee ST, Nicholls RD, Jong MTC, Fukai K, Spritz RA. Organization and sequence of the human P gene and identification of a new family unit of send proteins. Genomics. 1995;26:354–63.
-
Edwards M, Bigham A, Tan J, Li S, Gozdzik A, Ross K, et al. Association of the OCA2 polymorphism His615Arg with melanin content in Due east Asian populations: farther show of convergent evolution of pare pigmentation. PLoS Genet. 2010;6:e1000867.
-
Abe Y, Tamiya G, Nakamura T, Hozumi Y, Suzuki T. Clan of melanogenesis genes with pare color variation amongst Japanese females. J Dermatol Sci. 2013;69:167–72.
-
Alonso S, Izagirre Northward, Smith-Zubiaga I, Gardeazabal J, Díaz-Ramón JL, Díaz-Pérez JL, et al. Complex signatures of selection for the melanogenic loci TYR, TYRP1 and DCT in humans. BMC Evol Biol. 2008;8:74.
-
Lao O, De Gruijter JM, Van Duijn K, Navarro A, Kayser Thousand. Signatures of positive selection in genes associated with human pare pigmentation every bit revealed from analyses of single nucleotide polymorphisms. Ann Hum Genet. 2007;71:354–69.
-
Williamson SH, Hubisz MJ, Clark AG, Payseur BA, Bustamante CD, Nielsen R. Localizing contempo adaptive evolution in the human genome. PLoS Genet. 2007;3:e90.
-
Pickrell JK, Coop G, Novembre J. Signals of recent positive selection in a worldwide sample of human populations. Genome Res. 2009;19:826–37.
-
Wehrle-Haller B. The part of kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Res. 2003;sixteen:287–96.
-
Imokawa Thou. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17:96–110.
-
Bennett DC, Lamoreux ML. The colour loci of mice--a genetic century. Paint Cell Res. 2003;sixteen:333–44.
-
Miller CT, Beleza Southward, Pollen AA, Schluter D, Kittles RA, Shriver MD, et al. Cis-regulatory changes in kit ligand expression and parallel development of pigmentation in sticklebacks and humans. Cell. 2007;131:1179–89.
-
Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, et al. Genetic determinants of hair, heart and peel pigmentation in Europeans. Nat Genet. 2007;39:1443–52.
-
Zhang M, Vocal F, Liang L, Nan H, Zhang J, Liu H, et al. Genome-wide association studies identify several new loci associated with pigmentation traits and skin cancer take a chance in European Americans. Hum Mol Genet. 2013;22:2948–59.
-
Van Der Harst P, Zhang Due west, Leach IM, Rendon A, Verweij N, Sehmi J, et al. Seventy-v genetic loci influencing the man red claret cell. Nature. 2012;492:369–75.
-
Lin BD, Mbarek H, Willemsen K, Dolan CV, Fedko IO, Abdellaoui A, et al. Heritability and genome-broad clan studies for hair color in a Dutch twin family based sample. Genes. 2015;6:559–76.
-
Santos M, Mcevoy B, Alves I, Cameron Eastward, Shriver MD, Parra EJ, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;thirty:24–35.
-
Bonilla C, Boxill L-A, McDonald SA, Williams T, Sylvester N, Parra EJ, et al. The 8818G allele of the agouti signaling protein (ASIP) factor is ancestral and is associated with darker skin color in African Americans. Hum Genet. 2005;116:402–6.
-
Zeigler-johnson C, Panossian S, Gueye SM, Jalloh M, Ofori-Adjei D, Kanetsky PA. Population differences in the frequency of the agouti signaling poly peptide yard.8818A>G polymorphism. Paint Cell Res. 2004;17:185–vii.
-
Lu D, Lou H, Yuan Chiliad, Wang X, Wang Y, Zhang C, et al. Bequeathed origins and genetic history of Tibetan highlanders. Am J Hum Genet. 2016;99:580–94.
-
Jin W. Admixture dynamics, natural selection and diseases in admixed populations. Netherlands: Springer; 2015.
-
Wang S, Ray N, Rojas W, Parra MV, Bedoya 1000, Gallo C, et al. Geographic patterns of genome admixture in Latin American mestizos. PLoS Genet. 2008;4:e1000037.
-
Deng L, Ruiz-Linares A, Xu Southward, Wang S. Beginnings variation and footprints of natural selection forth the genome in Latin American populations. Sci Rep. 2016;6:21766.
-
Beleza S, Johnson NA, Candille SI, Absher DM, Coram MA, Anderson TM, et al. Genetic architecture of skin and eye colour in an African-European admixed population. PLoS Genet. 2013;nine:e1003372.
-
Shriver Md, Parra EJ, Dios Southward, Bonilla C, Norton H, Jovel C, et al. Peel pigmentation, biogeographical ancestry and admixture mapping. Hum Genet. 2003;112:387–99.
-
Bonilla C, Shriver MD, Parra EJ, Jones A, Fernández JR. Ancestral proportions and their clan with skin pigmentation and bone mineral density in Puerto Rican women from New York city. Hum Genet. 2004;115:57–68.
-
Kanetsky PA, Swoyer J, Panossian S, Holmes R, Guerry D, Rebbeck TR. A polymorphism in the agouti signaling protein gene is associated with human being pigmentation. Am J Hum Genet. 2002;70:770–five.
-
de Araújo LF, de Toledo GF, Fridman C. SLC24A5 and ASIP as phenotypic predictors in Brazilian population for forensic purposes. Legal Med. 2015;17:261–6.
-
Bonilla C, Parra EJ, Pfaff CL, Dios S, Marshall JA, Hamman RF, et al. Admixture in the Hispanics of the San Luis Valley, Colorado, and its implications for complex trait gene mapping. Ann Hum Genet. 2004;68:139–53.
-
Stokowski RP, Krishna Pant VK, Dadd T, Fereday A, Hinds DA, Jarman C, et al. A genomewide association report of skin pigmentation in a south Asian population. Am J Hum Genet. 2007;81:1119–32.
-
Basu Mallick C, Iliescu FM, Möls M, Hill S, Tamang R, Chaubey K, et al. The light skin allele of SLC24A5 in south Asians and Europeans shares identity by descent. PLoS Genet. 2013;9:e1003912.
-
Cavalli-Sforza LL, Menozzi P PA. The history and geography of human genes. Princeton, NJ Princet. Univ. Press. 1994.
-
Endicott P. Introduction: revisiting the "negrito" hypothesis: a transdisciplinary approach to man prehistory in Southeast Asia. Hum Biol. 2013;85:vii–xx.
-
Manni F, Toupance B, Migliano AB, Romero IG, Metspalu M, Leavesley K, et al. Evolution of the pygmy phenotype: evidence of positive pick from genome-wide scans in African, Asian, and Melanesian pygmies. Hum Biol. 2013;85:251–84.
-
Clavano-Harding AB, Ambler GR, Cowell CT, Garnett SP, Al-Toumah B, Coakley JC, et al. Initial characterization of the GH-IGF axis and nutritional status of the Ati Negritos of the Philippines. Clin Endocrinol. 1999;51:741–vii.
-
Dávila N, Shea BT, Omoto K, Mercado M, Misawa Southward, Baumann Yard. Growth hormone binding protein, insulin-like growth gene-I and short stature in two pygmy populations from the Philippines. J Pediatr Endocrinol Metab. 2002;15:269–76.
-
Mendizabal I, Marigorta UM, Lao O, Comas D. Adaptive evolution of loci covarying with the human African pygmy phenotype. Hum Genet. 2012;131:1305–17.
-
Norton HL, Werren Due east, Friedlaender J. MC1R multifariousness in Northern Island Melanesia has not been constrained by strong purifying selection and cannot explain pigmentation phenotype variation in the region. BMC Genet. 2015;16:122.
-
Rana BK, Hewett-Emmett D, Jin L, Chang BHJ, Sambuughin N, Lin Thou, et al. High polymorphism at the human being melanocortin 1 receptor locus. Genetics. 1999;151:1547–57.
-
Norton HL, Friedlaender JS, Merriwether DA, Koki M, Mgone CS, Shriver MD. Skin and pilus pigmentation variation in island Melanesia. Am J Phys Anthropol. 2006;130:254–68.
-
Kenny EE, Timpson NJ, Sikora M, Yee MC, Moreno-Estrada A, Eng C, et al. Melanesian blond hair is caused by an amino acid change in TYRP1. Science 2012;336:554–554.
-
Norton HL, Correa EA, Koki G, Friedlaender JS. Distribution of an allele associated with blond hair color beyond northern island melanesia. Am J Phys Anthropol. 2014;153:653–62.
-
Skoglund P, Posth C, Sirak One thousand, Spriggs M, Valentin F, Bedford S, et al. Genomic insights into the peopling of the Southwest Pacific. Nature. 2016;538:510–3.
-
Delfin F, Myles S, Choi Y, Hughes D, Illek R, van Oven Grand, et al. Bridging most and remote Oceania: mtDNA and NRY variation in the Solomon Islands. Mol Biol Evol. 2012;29:545–64.
-
Friedlaender JS, Friedlaender FR, Reed FA, Kidd KK, Kidd JR, Chambers GK, et al. The genetic structure of Pacific islanders. PLoS Genet. 2008;iv:e19.
-
Ang KC, Ngu MS, Reid KP, Teh MS, Aida ZS, Koh DX, et al. Skin color variation in Orang Asli tribes of peninsular Malaysia. PLoS One. 2012;7:e42752.
-
Schaebel LK, Bonefeld-Jørgensen EC, Laurberg P, Vestergaard H, Andersen S. Vitamin D-rich marine Inuit diet and markers of inflammation–a population-based survey in Greenland. J Nutr Sci. 2015;4:e40.
-
Kolahdooz F, Barr A, Roache C, Sheehy T, Corriveau A, Sharma S. Dietary capability of vitamin D and calcium among inuit and inuvialuit women of kid-begetting age in Arctic Canada: a growing business organisation. PLoS One. 2013;eight:e78987.
-
Jablonski NG, Chaplin M. The evolution of human being skin coloration. J Hum Evol. 2000;39:57–106.
-
Rogers AR, Iltis D, Wooding S. Genetic variation at the MC1R locus and the fourth dimension since loss of human body hair. Curr Anthropol. 2003:105–8.
-
Branda RF, Eaton JW. Skin color and nutrient photolysis: an evolutionary hypothesis. Scientific discipline. 1978;201:625–half-dozen.
-
Robins AH. Biological Perspectives on Human Pigmentation. Cambridge: Cambridge Univeristy Press; 1991.
-
Peter BM, Huerta-Sanchez E, Nielsen R. Distinguishing betwixt selective sweeps from standing variation and from a de novo mutation. PLoS Genet. 2012;8:e1003011.
-
Mathieson I, Lazaridis I, Rohland N, Mallick S, Patterson N, Roodenberg SA, et al. Genome-broad patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499–503.
-
Wilde S, Timpson A, Kirsanow K, Kaiser E, Kayser M, Unterländer M, et al. Straight evidence for positive choice of peel, hair, and heart pigmentation in Europeans during the last 5,000 y. Proc Natl Acad Sci U S A. 2014;111:4832–7.
-
Richards MP, Schulting RJ, Hedges RE. Archaeology: abrupt shift in diet at onset of Neolithic. Nature. 2003;425:366.
-
Olalde I, Allentoft ME, Sánchez-Quinto F, Santpere Grand, Chiang CWK, DeGiorgio M, et al. Derived allowed and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature. 2014;507:225–eight.
-
Finlayson C, Pacheco FG, Rodríguez-Vidal J, Fa DA, Gutierrez López JM, Santiago Pérez A, et al. Late survival of Neanderthals at the southernmost extreme of Europe. Nature. 2006;443:850–3.
-
Durand EY, Patterson Northward, Reich D, Slatkin M. Testing for aboriginal admixture between closely related populations. Mol Biol Evol. 2011;28:2239–52.
-
Fu Q, Hajdinjak M, Moldovan OT, Constantin South, Mallick Southward, Skoglund P, et al. An early mod human from Romania with a recent Neanderthal ancestor. Nature. 2015;524:216–9.
-
Fu Q, Li H, Moorjani P, Jay F, Slepchenko SM, Bondarev AA, et al. Genome sequence of a 45,000-year-one-time mod human from western Siberia. Nature. 2014;514:445–50.
-
Vernot B, Akey JM. Resurrecting surviving Neandeltal linages from modern human genomes. Science. 2014;343:1017–21.
-
Ding Q, Hu Y, Xu S, Wang C, Li H, Zhang R, et al. Neanderthal origin of the haplotypes carrying the functional variant Val92Met in the MC1R in modern humans. Mol Biol Evol. 2014;31:1994–2003.
-
Ding Q, Hu Y, Xu S, Wang J, Jin L. Neanderthal introgression at chromosome 3p21.31 was under positive natural selection in Eastward Asians. Mol. Biol. Development. 2013;31:683–95.
-
Sankararaman S, Mallick Southward, Dannemann M, Prüfer K, Kelso J, Pääbo S, et al. The landscape of Neandertal ancestry in present-twenty-four hours humans. Nature. 2014;507:354–vii.
-
Cerqueira CCS, Paixão-Côrtes VR, Zambra FMB, Salzano FM, Hünemeier T, Bortolini MC. Predicting homo pigmentation phenotype through genomic data: from neanderthal to James Watson. Am J Hum Biol. 2012;24:705–ix.
-
Lalueza-Play a trick on C, Römpler H, Caramelli D, Stäubert C, Catalano G, Hughes D, et al. A melanocortin ane receptor allele suggests varying pigmentation among Neanderthals. Science. 2007;318:1453–v.
-
López South, García Ó, Yurrebaso I, Flores C, Acosta-Herrera M, Chen H, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PLoS One. 2014;9:e104367.
-
Itan Y, Powell A, Beaumont MA, Burger J, Thomas MG. The origins of lactase persistence in Europe. PLoS Comput Biol. 2009;5:e1000491.
-
Gerbault P, Moret C, Currat M, Sanchez-Mazas A. Impact of selection and demography on the diffusion of lactase persistence. PLoS One. 2009;4:e6369.
-
Tishkoff SA, Varkonyi R, Cahinhinan North, Abbes S, Argyropoulos Thousand, Destro-Bisol One thousand, et al. Haplotype diversity and linkage disequilibrium at human G6PD: recent origin of alleles that confer malarial resistance. Science. 2001;293:455–62.
-
Kamberov YG, Wang S, Tan J, Gerbault P, Wark A, Tan 50, et al. Modeling recent homo development in mice past expression of a selected EDAR variant. Prison cell. 2013;152:691–702.
-
Jeong C, Alkorta-Aranburu Thou, Basnyat B, Neupane M, Witonsky DB, Pritchard JK, et al. Admixture facilitates genetic adaptations to loftier altitude in Tibet. Nat Commun. 2014;five:3281.
-
Sturm RA. Molecular genetics of human pigmentation multifariousness. Hum Mol Genet. 2009;18:ix–17.
-
Coop G, Pickrell JK, Novembre J, Kudaravalli S, Li J, Absher D, et al. The function of geography in human adaptation. PLoS Genet. 2009;5:e1000500.
-
Flanagan N, Healy Eastward, Ray A, Philips S, Todd C, Jackson IJ, et al. Pleiotropic effects of the melanocortin 1 receptor (MC1R) factor on human pigmentation. Hum Mol Genet. 2000;nine:2531–vii.
-
Córdoba-Lanús Due east, Hernández-Jiménez JG, Medina-Coello C, Espinoza-Jiménez A, González A, Rodríguez-Pérez MDC, et al. MC1R gene variants and sporadic malignant melanoma susceptibility in the Canary Islands population. Arch Dermatol Res. 2014;306:51–8.
-
Yang Z, Zhong H, Chen J, Zhang X, Zhang H, Luo Ten, et al. A genetic mechanism for convergent peel lightening during recent human being evolution. Mol Biol Evol. 2016;33:1177–87.
Acknowledgements
We thank LetPub (world wide web.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
S.Ten. acknowledges fiscal support from the National Natural Science Foundation of Red china (NSFC) grant (91331204 and 31711530221), the Strategic Priority Research Programme (XDB13040100) and Key Research Program of Frontier Sciences (QYZDJ-SSW-SYS009) of the Chinese Academy of Sciences (CAS), the National Science Fund for Distinguished Immature Scholars (31525014), and the Program of Shanghai Academic Research Leader (16XD1404700); S.Ten. is Max-Planck Contained Research Group Leader and member of CAS Youth Innovation Promotion Clan. Southward.Ten. also gratefully acknowledges the support of the National Program for Height-notch Young Innovative Talents of The "Wanren Jihua" Project. The funders had no function in study design, data collection and assay, decision to publish, or preparation of the manuscript.
Availability of data and materials
Not applicative.
Authors' contributions
SX and LD drafted the manuscript. Both authors read and approved the last manuscript.
Competing interests
The authors declare that they have no competing interests.
Ideals approval and consent to participate
Not applicable.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author data
Affiliations
Respective author
Rights and permissions
Open Access This commodity is distributed under the terms of the Creative Eatables Attribution 4.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted use, distribution, and reproduction in whatsoever medium, provided y'all requite appropriate credit to the original writer(s) and the source, provide a link to the Creative Commons license, and point if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Deng, L., Xu, S. Adaptation of human being pare colour in diverse populations. Hereditas 155, 1 (2018). https://doi.org/ten.1186/s41065-017-0036-2
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/ten.1186/s41065-017-0036-ii
Keywords
- Skin color
- Natural selection
- Genetic accommodation
- Modern humans
- Primitive hominin
Source: https://hereditasjournal.biomedcentral.com/articles/10.1186/s41065-017-0036-2
Posted by: smithplagne.blogspot.com

0 Response to "What Is A Hypothesis On Natural Selection Affects Skin Color"
Post a Comment